General Information
| Clinical Research Resources (CRR) |
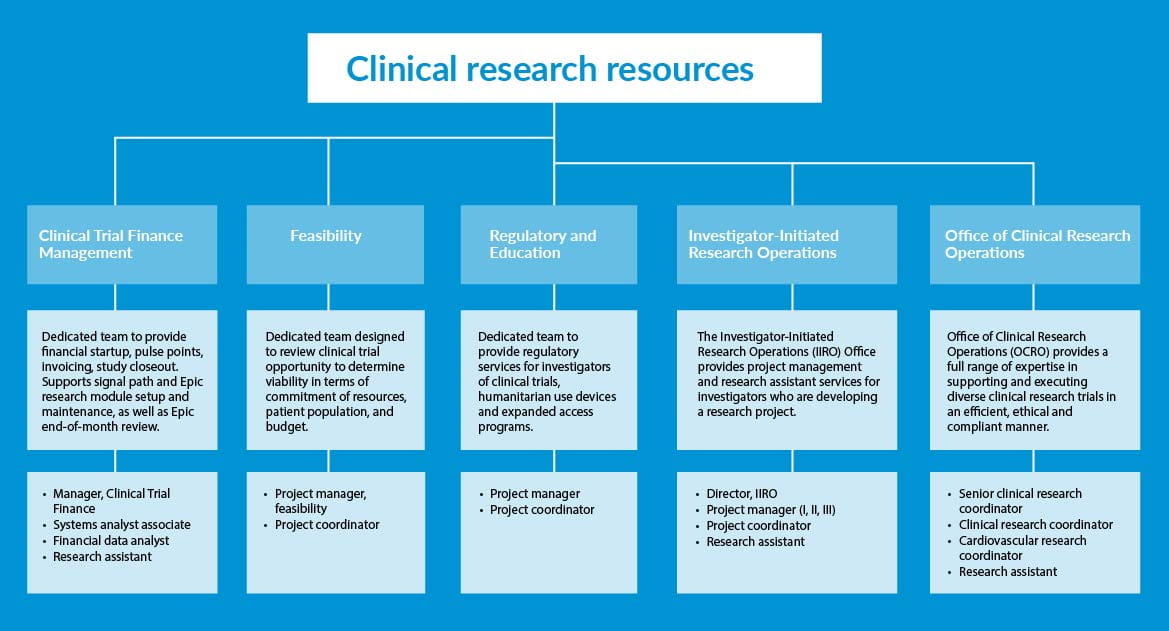
Clinical Research Resources includes five research cores at Geisinger:
- Office of Clinical Research Operations (OCRO)
- Investigator Initiated Research Operations
- Clinical Trial Finance
- Feasibility
- Regulatory and Education
|
| Audits and Inspections |
No 483 warning letters issued from three FDA audits over the past 10 years |
| OCRO SOPs and Best Practices |
SOPs available upon request |
| Staff |
- More than a decade of experience managing trials in multiple therapeutic areas-cardiovascular, pulmonary, gastroenterology, endocrine, OB/GYN, palliative care, critical care, laboratory medicine, general internal medicine
- More than 90% of Clinical Research Coordinators (CRC) are RNs
- All CRC’s and Project Managers are required to be SOCRA and/or ACRP certified
- All study staff and investigators are required to complete and maintain Basic Human Subjects, Responsible Conduct for Research and Good Clinical Practice training through the Collaborative Institutional Training Initiative (CITI)
|
| Study Monitors |
- Wireless Internet available
- CRO & Sponsor Monitors are provided with laptop and access to subjects’ EMR; 2 weeks advance notice needed for access approval
- Space provided for monitoring visits
- Remote monitoring access available with provisions
|
| Different Types of Sponsor Experience/Funding Sources |
- Industry
- NIH, NIDDK, NHLBI, Foundations
- Investigator Initiated
|
| Trial Phase Capabilities |
II, III, IV |
| Miscellaneous |
- Epic: EMR 21 CFR Part II compliant
- Dedicated computers for each staff member
- High speed internet access
- Internet browser: Internet Explorer Version 11.0
- Prompt reporting of inpatient hospitalization SAEs through a unique process which alerts study staff to participants’ in-system hospitalizations on a daily basis
|
| Long Term Storage |
GSS Storage Facility
400 Railroad St., Danville, PA 17821
|
Recruitment
| Mature Electronic Medical Records |
- Contains information on > 4 million unique patients
- Records provide real time and historical data useful in pre-identifying potentially eligible study patients
- Allows access for provider referrals in multiple therapeutic areas
|
| Large Trial Recruitment Process |
- Using the Electronic Medical Record (EMR), data pulls specific to study inclusion/exclusion criteria are programmed primarily by ICD and procedure codes
- Identified eligible patients are invited to learn more about the study
- Potential patients are listed on a study tracker and contacted by staff
- Our process has shown to deliver high enrollment rates, realistic enrollment expectations and low screen failure rates
|
| Dedicated Research Data & Recruitment Manager |
- Oversees implementation of trial recruitment strategies and enrollment optimization
- Facilitates management of data requests, externally and internally
|
| Excellent Retention Rates |
- The experience and dedication of OCRO staff, convenient patient tools and a low out-migration rate results in excellent retention rates that are well above average.
- OCRO has a 10+ year history of conducting 3–5-year outcomes trials with excellent retention rates over 95%
|
| MyGeisinger.org |
- MyGeisinger.org: A patient web-based portal that provides a convenient way for patients to access their medical record, contact providers/study staff and manage their health.
- MyChart Mobile: This app provides a MyGeisinger user with secure access to features such as messaging providers, viewing your own and family medical records, upcoming and past appointments, test results, etc. These tools are helpful with both recruitment and retention of study patients.
|
Regulatory Information
|
Local Institutional Review Board
(Geisinger IRB - GIRB)
Phone: 570-271-8663
Fax: 570-214-7031
|
- Full Board review occurs twice monthly. Submission deadline for board review required approximately 3 weeks prior to meeting date
- Exempt and Expedited submissions take approximately 2-3 weeks for review and approval
- Full Board submissions typically take 1-2 months from submission to approval
- GIRB uses iRIS, an electronic document platform for all submissions and tracking
- The IRB consists of 11 members, 2 co-chairs, 2 ex-officio members, plus 12 alternates
- GIRB is fully accredited by AAHRPP
|
| HHS Federal-wide Assurance Protection for Human Subjects |
Number: FWA00000063
Expires: 17SEP2027 |
| Regulatory and IRB Support |
- One Project Manager dedicated to supporting regulatory tasks for the group
- IRB submissions, amendments, communications and tracking completed centrally for GHS OCRO through this central support service
|
| IRB Fees |
A one-time fee will be charged for IRB review of all externally-funded research studies. The IRB fee covers initial review and all continuing reviews, amendments and unanticipated problems submitted to the IRB for the life of the study. |
Contracts/Budget
- Centralized team responsible for CDA’s, agreements, contract & budget negotiation at all Geisinger sites
- Average timeline for contract execution: 3-4 months
- Current overhead and applicable fees will apply as appropriate
- Contracting Entity: Geisinger Clinic (for all Geisinger sites)
Payee & Address:
Geisinger Clinic
Attn: Research Finance, MC 30-69
100 N. Academy Avenue
Danville, PA 17822-3069
researchach@geisinger.edu
Point of Contact:
Candice Laubach, MBA, CRCP (or designee assigned)
Vice President, Research
Geisinger Clinic
100 N. Academy Ave
Danville, PA 17822-4400
researchcontracts@geisinger.edu
Phone: 570-214-5067
Geisinger Medical Center, Danville PA
| Address |
100 North Academy Avenue
Danville, PA 17822-4400 |
| Office of Clinical Research Operations |
Phone: 570-214-1186
Fax: 570-271-6944 |
| Type of Institution |
Academic Medical Center and Outpatient Specialty Clinics
|
| Number of Beds North Central Region |
560 beds at Geisinger Medical Center (GMC)
72 beds at Geisinger Bloomsburg Hospital
55 beds at Geisinger Shamokin Area Hospital
123 beds at Geisinger Lewistown
311 beds at Holy Spirit Hospital |
| Research Staffing |
14 Clinical Research Coordinators
- 10 Registered Nurses
- 4 Cardiovascular Research Coordinators
7 Research Assistants
|
| Investigational Pharmacy |
Contact: Adam Gross, PharmD, CCRP
Coordinator – Investigational Drug Service
Enterprise Pharmacy
100 Academy Avenue
Danville, PA 17822-4201
Services & Equipment
- Drug procurement, preparation & labeling
- Secure, monitored storage
- Accountability (Vestigo)
- Storage room and refrigerator equipped with the TempTrak 24-hour monitoring and alarm system. Traceable to NIST standards.
- Monthly digital temperature records accessible to study staff/sponsors
- Refrigerator connected to the hospital’s back-up generator system
- Disposal of study drug; incineration at off-campus sites
|
| Local Laboratory |
Contact: Mike Weaver
Analytical Specialist
Referred Lab Testing
Referred Testing Staff all IATA Certified
CLIA and CAP certificates available
Services & Equipment
- Manages collection and processing of research samples (blood or tissue)
- Packaging and shipment of samples outside of Geisinger
- Research sample storage, refrigerated or frozen
- Freezers and refrigerators used to store samples equipped with electronic TempTrak 24-hour monitoring and alarm system.
- Monthly temperature records accessible to study staff/sponsors
- Refrigerators/freezers connected to the hospital’s back-up generator system
- Equipment calibration/validation completed annually with records accessible to study staff
- -20 and -70 degree C freezers
|
| On site in-patient and out-patient facilities and equipment |
ECG machines, scales, blood pressure cuffs and sphygmomanometers, otoscopes.
DEXA, X-ray, MRI, CT, echo, etc.
All equipment calibrated every 6 months |
Geisinger Wyoming Valley Medical Center
| Address |
1000 East Mountain Boulevard
Wilkes-Barre, PA 18711 |
| Research Office Phone Number |
Phone: 570-808-1001 |
| Type of Institution |
Academic Medical Center and Outpatient Specialty Clinics |
| Number of Beds |
274 |
| Research Staffing |
5 Clinical Research Coordinators
- 4 Registered Nurses
- 1 Cardiovascular Research Coordinator
2 Research Assistant
|
| Investigational Pharmacy |
Contact: Karla Fleury, R.Ph
Clinical Pharmacist
Phone: 570-808-7704
Services & Equipment
Same as Geisinger Medical Center above |
| Local Laboratory (Hospital Based) |
Contact: Johnna Stepanek
Research Lab Assistant
Phone: 570-808-6091
Fax: 570-808-6090
Services & Equipment
Same as Geisinger Medical Center above |
| On-site in-patient and out-patient facilities and equipment |
ECG machines, scales, blood pressure cuffs and sphygmomanometers, otoscopes.
DEXA, X-ray, MRI, CT, echo, etc.
All equipment calibrated every 6 months |